barostim neo system
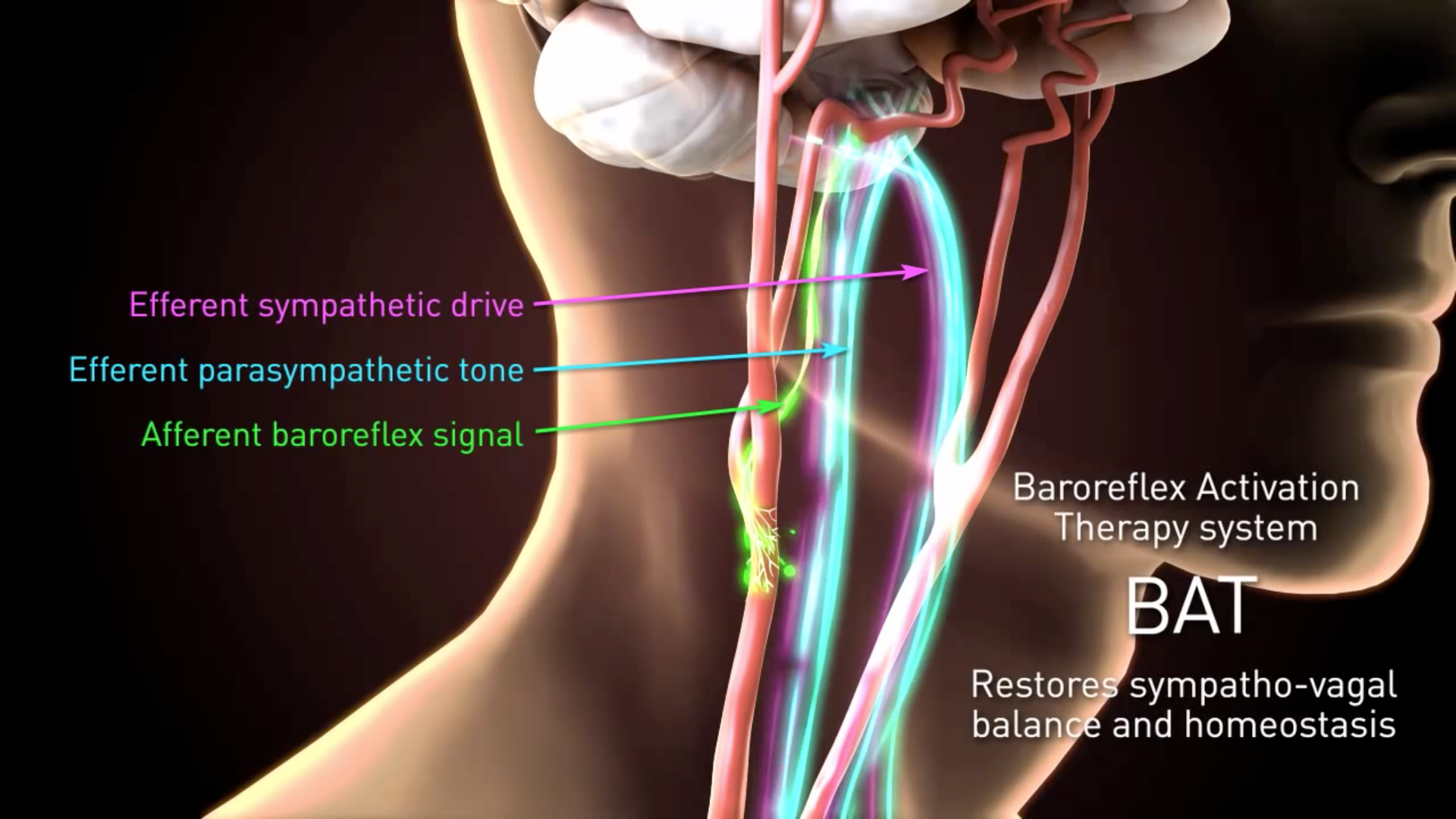
In the model Barostim reduced. Barostim neois designed to restore sympatho-vagal balance by activating afferent and efferent pathways of the autonomic nervous system to reduce excessive blood pressure and improve.

Ce Mark Approval For Heart Failure Treatment Today S Medical Developments
Barostim improves quality of life.

. Barostim Baroreflex Activation Therapy BAT uses the power of the autonomic nervous system to improve symptoms of heart failure. This pacemaker-like device is designed to. Barostim Neo - Baroreflex Activation Therapy for Heart Failure CMS Back to Approved IDE Studies Barostim Neo - Baroreflex Activation Therapy for Heart Failure Study.
The BAROSTIM NEO System is designed to electrically activate the carotid baroreceptors the bodys natural cardiovascular regulation sensors. The system received CE mark from the National Standards. CVRx a company out of Minneapolis Minnesota received CE Mark approval for its Barostim neo System thats intended for heart failure patients with an ejection fraction 35.
Anne Kroman Barostim won breakthrough device approval from the US. The BAROSTIM NEO System is indicated for subjects with heart failure defined as New York Heart Association NYHA functional Class III and left ventricular ejection fraction. The BAROSTIM NEO System includes the following components.
Barostim is a novel Congestive Heart Failure CHF treatment that uses the power of the brain to improve symptoms like breathlessness fatigue and swelling. BAROSTIM NEO is a US. When the baroreceptors are activated.
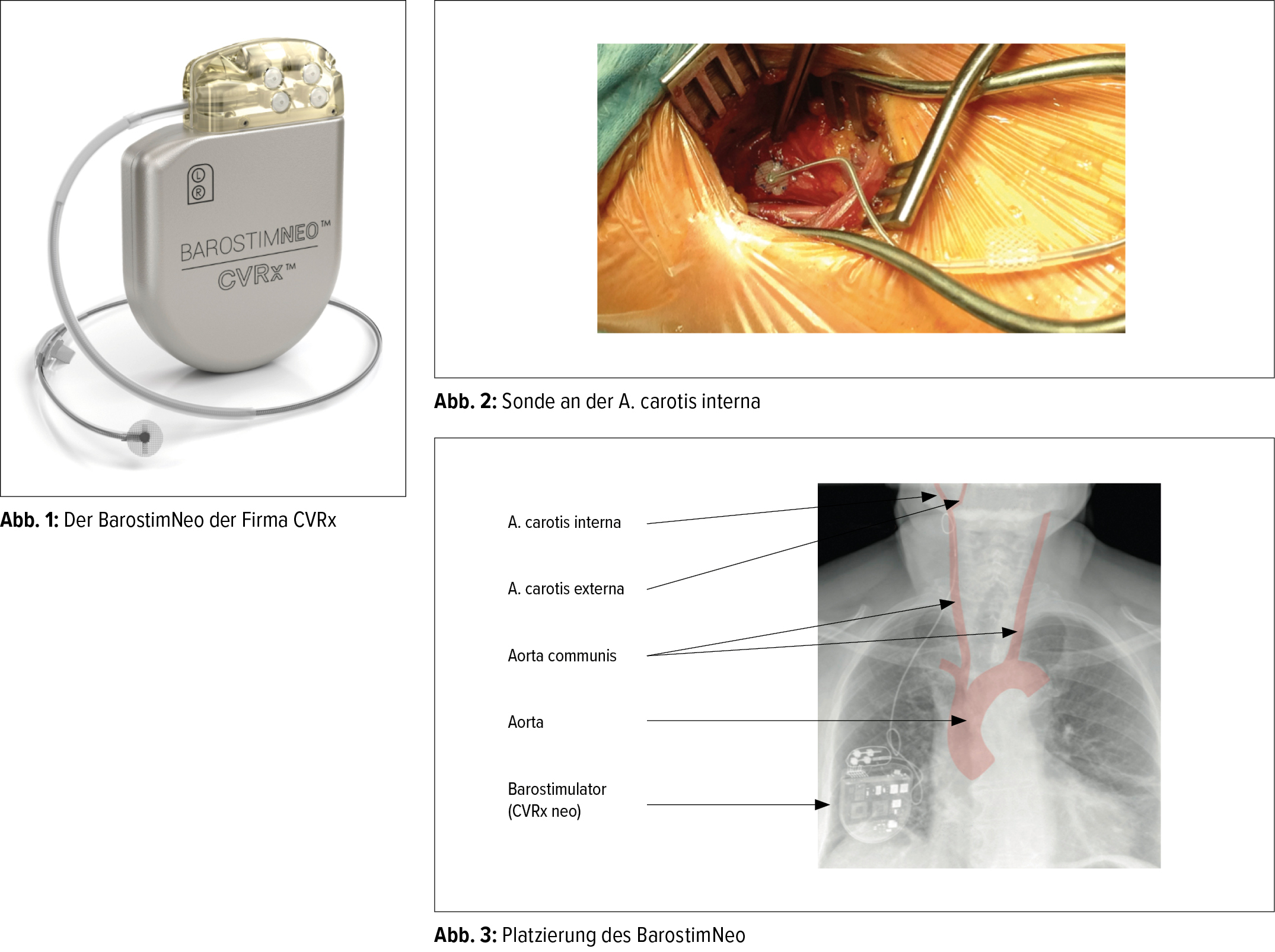
Device Models Implantable Pulse Generator IPG. IPG Torque Wrench Port Plug 2102 Carotid Sinus Lead. The minimally-invasive neo system uses CVRx patented Barostim Therapy technology to trigger.
The Barostim Neo System is indicated for patients who have a regular heart rhythm are not candidates for cardiac resynchronization therapy and have a left ventricular ejection fraction. The neo system is the CVRx next generation system for improving cardiovascular function. REGISTER for free or LOG IN to view this content.
Food and Drug Administration FDA-approved device that uses a novel mechanism to improve heart function. A prospective randomized study describing the safety and efficacy of the BAROSTIM NEO System in heart failure subjects with left ventricular ejection fraction equal to. The BAROSTIM NEO Hypertension Trial is a prospective randomized controlled trial assessing the safety and efficacy of the BAROSTIM NEO System in subjects with resistant.
The Barostim neo system is for patients having an ejection fraction less than or equal to 35 and a New York Heart Failure Classification of III without restriction on QRS. The Barostim System Baroreflex Activation Therapy is delivered by the BarostimNEO an implantable pulse generator IPG designed to deliver continuous electrical. Barostim was estimated to be cost-effective compared with optimal medical treatment with an incremental cost-effectiveness ratio of 7 797QALY.
The BAROSTIM NEO System Premarket Approval P180050 is a Class III carotid sinus stimulator an implantable medical device that delivers electrical signals to the bodys. Learn More Patient stories Im able to do the. Baroreflex Activation Therapy Barostim neo System Presenter.
The FDA approval for the CVRx Barostim Neo System is based on the data from a multi-center two-arm randomized clinical trial the BeAT-HF phase 3 study featuring 408. Barostim Neo is a neuromodulation system developed by CVRx for the treatment of heart failure and hypertension. Food and Drug Administration in 2019 following successful trials that were led by MUSC Health.
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

Baroreflex Activation Therapy Barostim Neo System Tctmd Com

Interventional Treatment For Heart Failure Receives Fda Approval Biba Medtech Insights

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram

Barostim Neo Neuromodulation Implantable System Usa
Barostim Neo 1 Radcliffe Cardiology

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram

Barostim Neo Neuromodulation Implantable System Usa

Innovative Device Therapie Der Herzinsuffizienz Management Krankenhaus

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram

Barostim Neo Neuromodulation Implantable System Usa

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice

Barostim Baroreflex Activation Therapy Cvrx

Investigational Device For Heart Failure Patients Stimulates Cells In Arteries To Improve Function Youtube
Baroreflex Aktivitatstherapie Bringt Funktionelle Verbesserung Bei Hi Patienten Kardiologie Gefassmedizin Universimed Medizin Im Fokus

Comments
Post a Comment